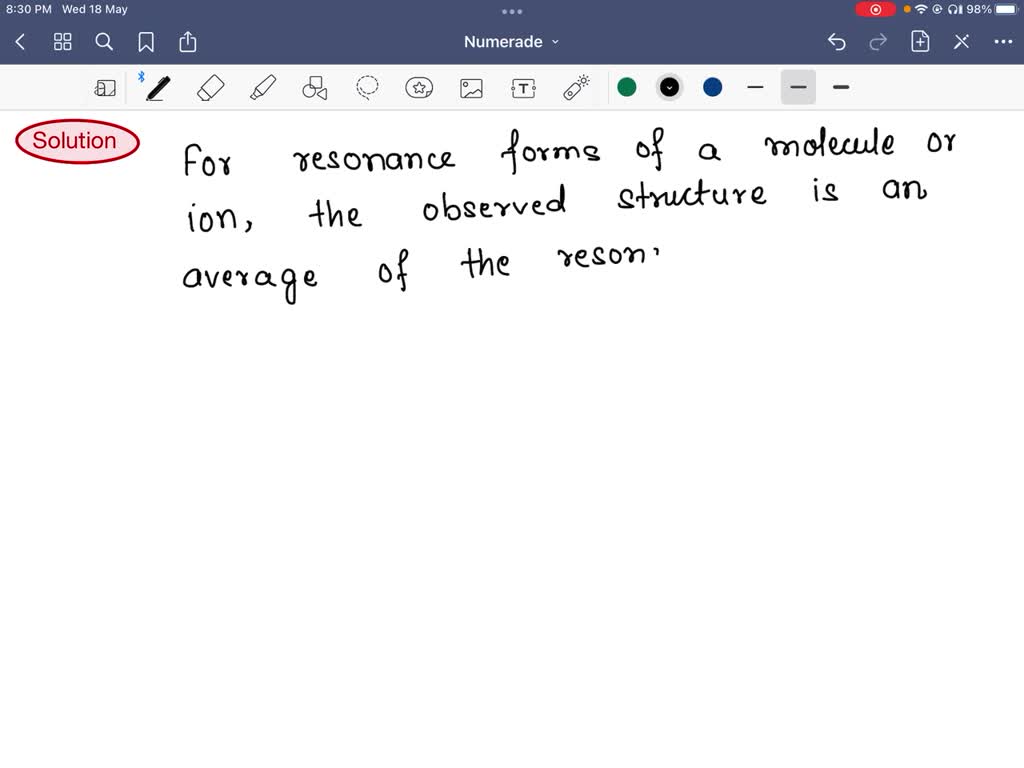
For Resonance Forms Of A Molecule Or Ion - The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
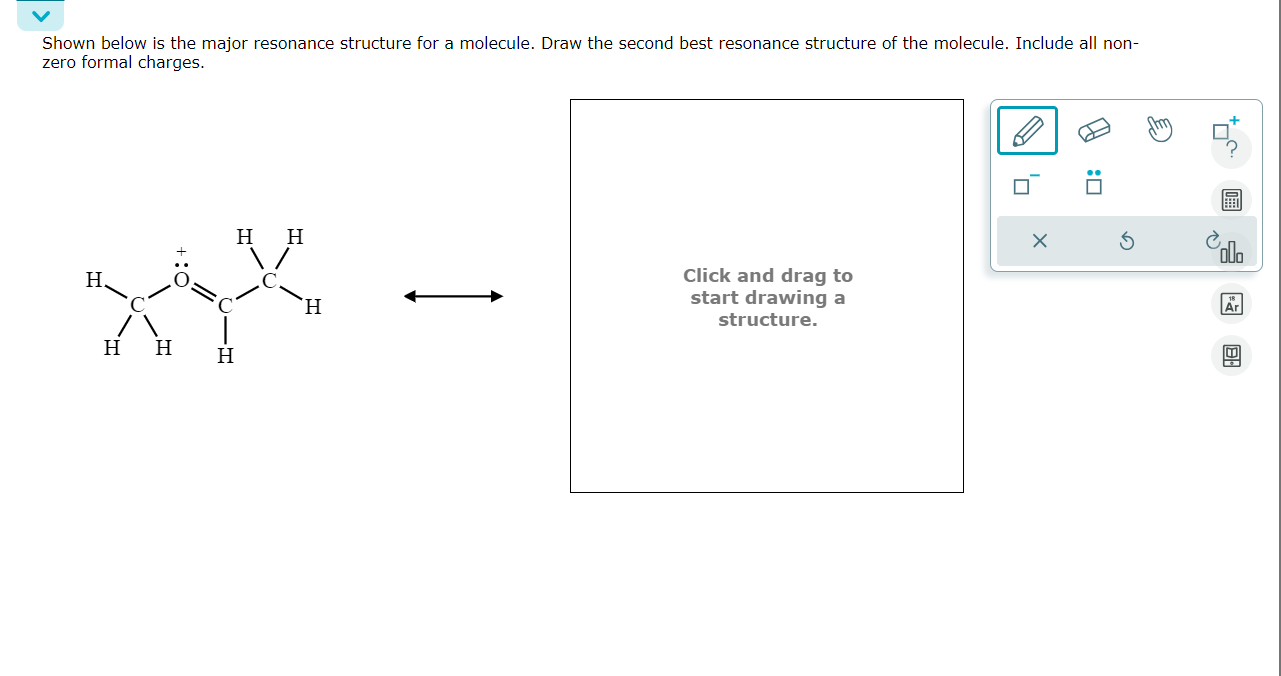
Solved Shown below is the major resonance structure for a
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
Solved Shown below is the major resonance structure for a
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
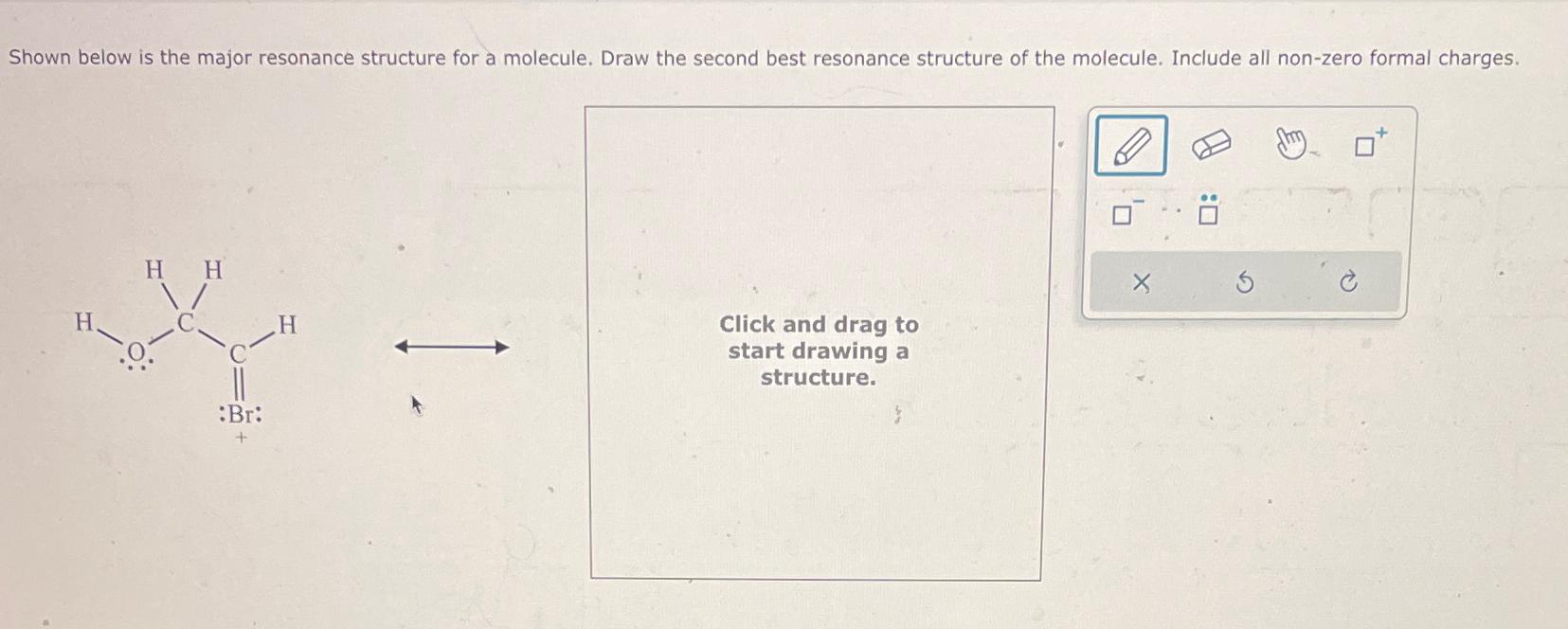
[Solved] Drawing resonance structure. Q2) Draw the MULTIPLE resonance
In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
[Solved] When a molecule has resonance forms, the most stable forms
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
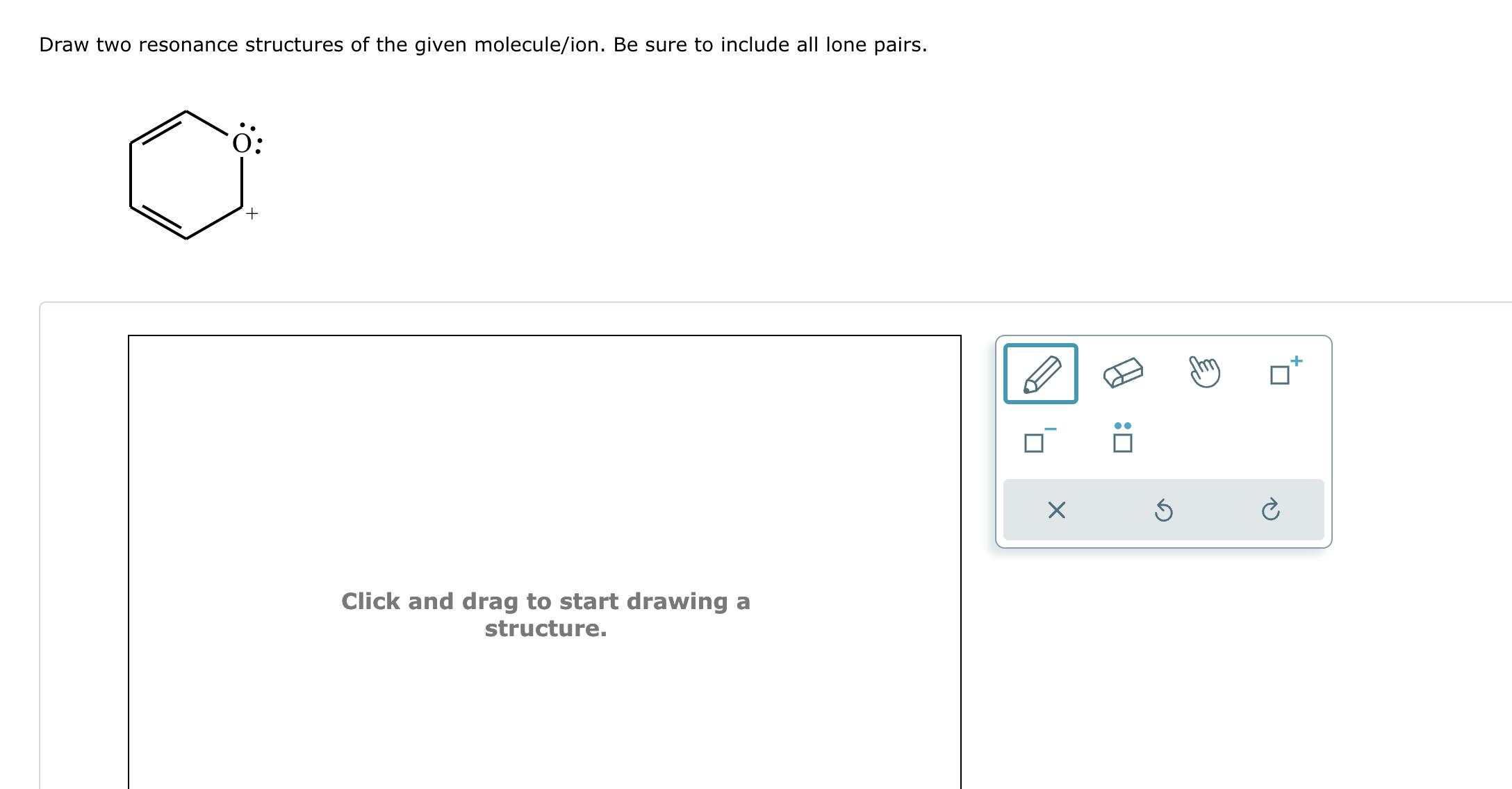
Solved Draw two resonance structures of the given
The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
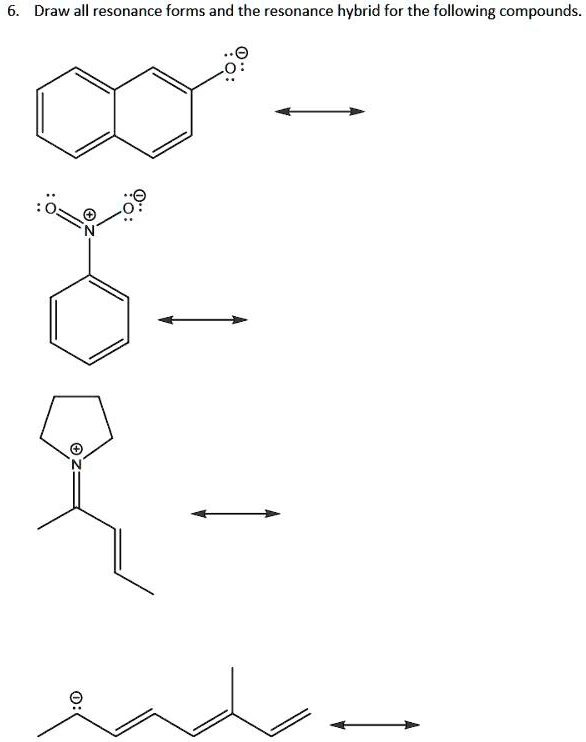
SOLVED Draw all resonance forms and the resonance hybrid for the
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
How to Know if a Molecule Has Resonance LeakruwBall
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. The actual structure is something in between the resonance structures and is known as a resonance hybrid.
SOLVED For resonance forms of a molecule or ion, one always
The actual structure is something in between the resonance structures and is known as a resonance hybrid. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
[Solved] Resonance forms Many molecules have resonance forms in which
A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or. The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.
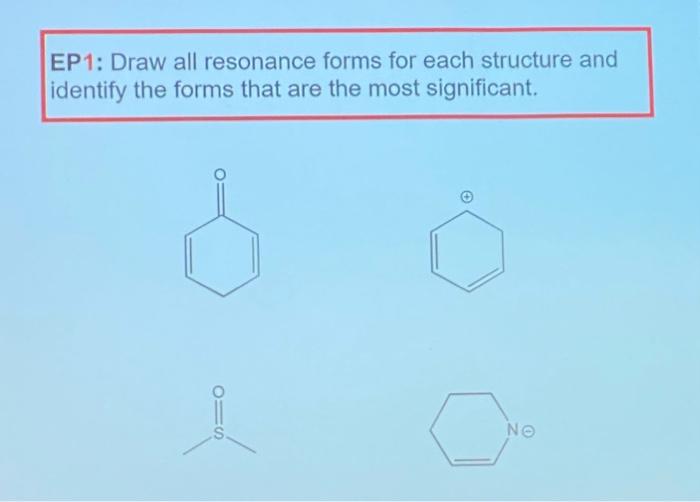
Solved EP1 Draw all resonance forms for each structure and
The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or.
A Molecule Or Ion With Such Delocalized Electrons Is Represented By Several Contributing Structures (Also Called Resonance Contributors Or.
The actual structure is something in between the resonance structures and is known as a resonance hybrid. In chemistry, resonance forms are different ways to depict the same molecule or ion that cannot be accurately described by a single.