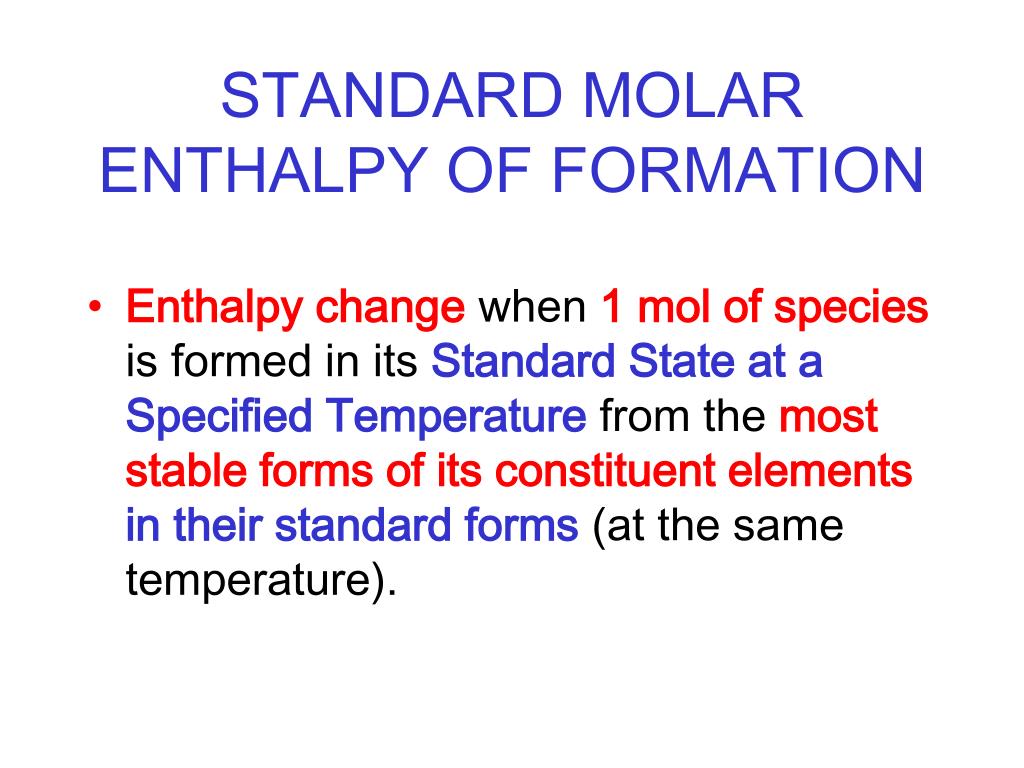
Standard Molar Internal Energy Of Formation - Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. How do you calculate standard molar enthalpy of formation?
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. How do you calculate standard molar enthalpy of formation?
How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with.
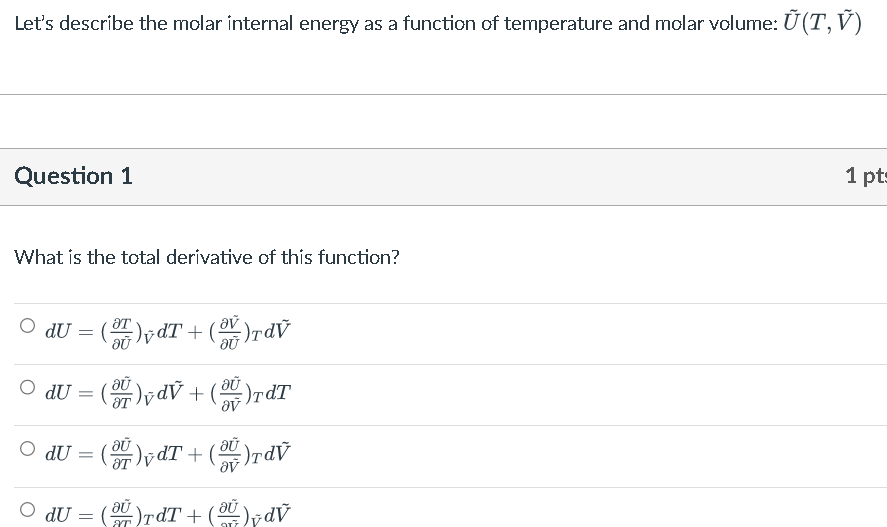
Solved Let's describe the molar internal energy as a
Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy.
Standard Molar Enthalpy of Formation answer
Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table.
Standard Molar Enthalpy of Formation Calculations A Chemistry
∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy.
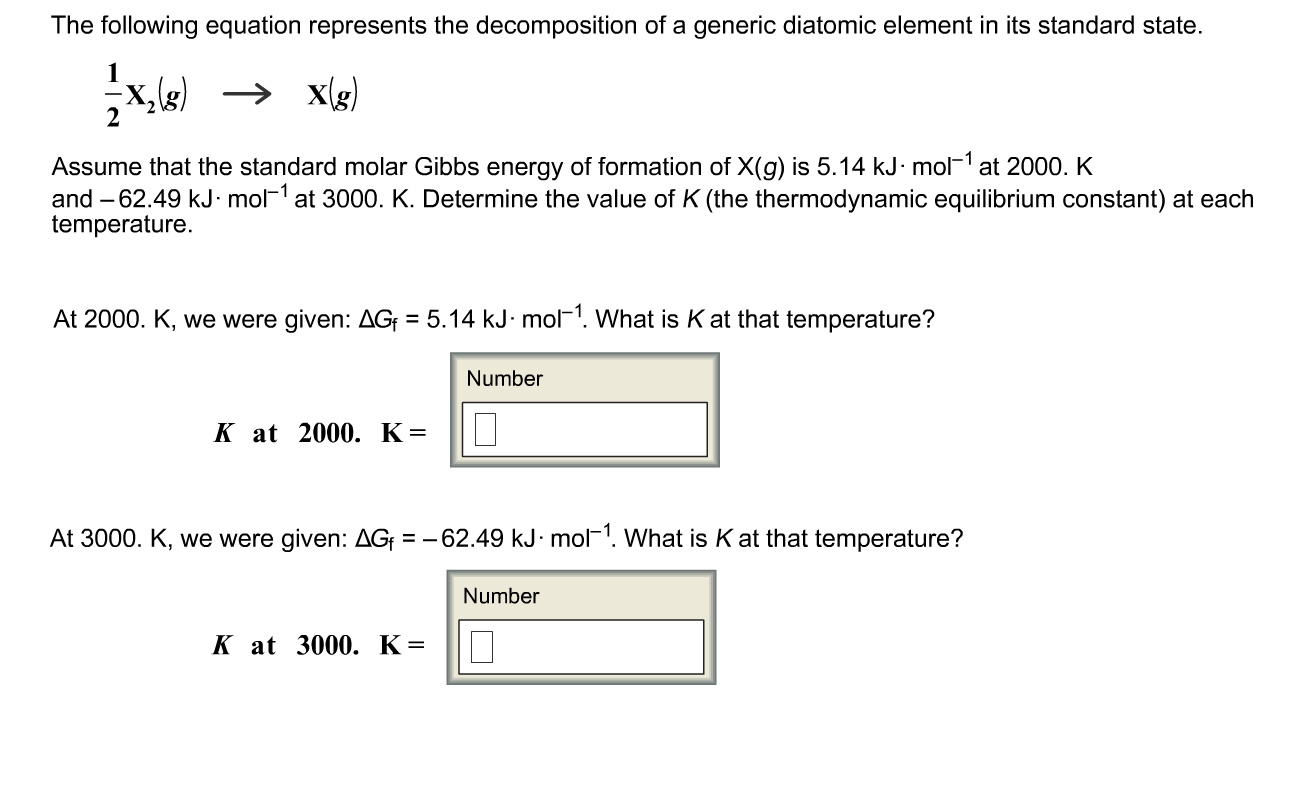
Solved Assume that the standard molar Gibbs energy of
How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy.
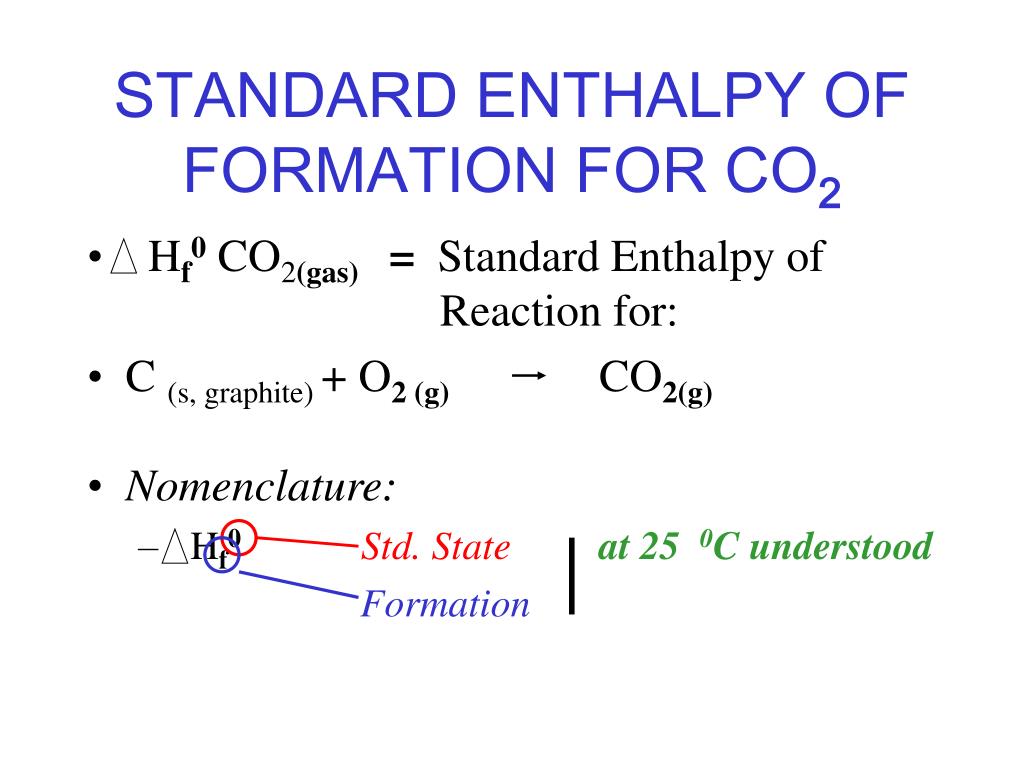
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy.
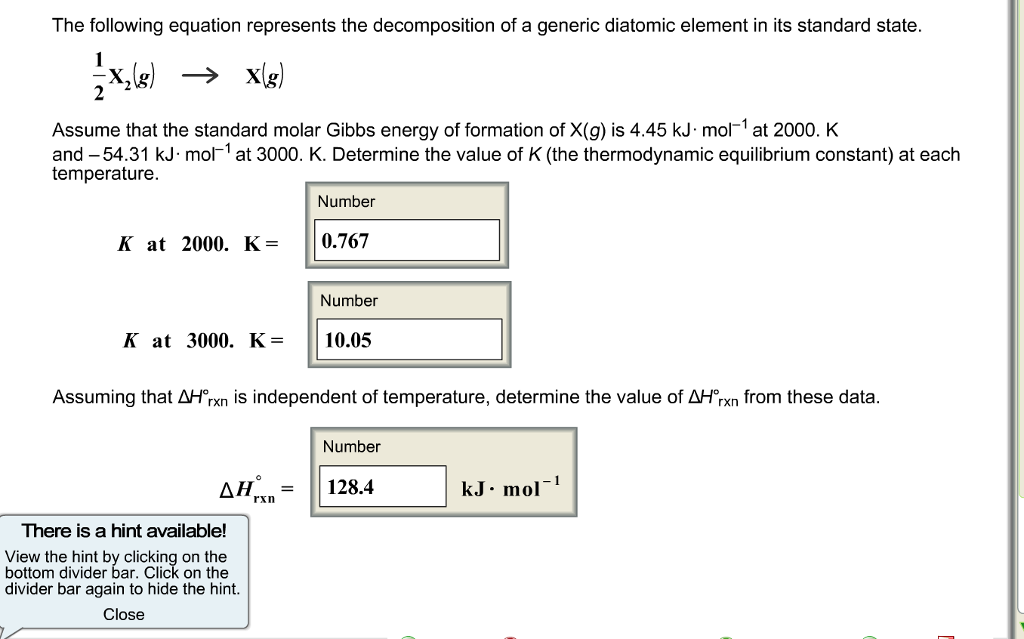
Solved Assume that the standard molar Gibbs energy of
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy.
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. How do you calculate standard molar enthalpy of formation? ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy.
Using Standard Molar Enthalpies of Formation to Calculate Enthalpy
How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. ∗the standard entropy of the h+(aq) ion is defined to be 0. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy.
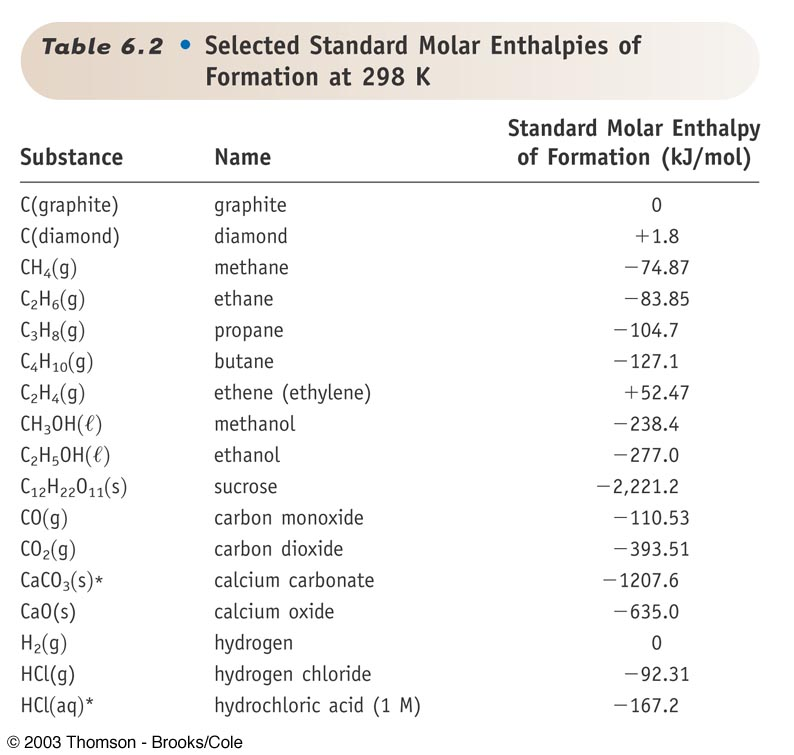
Solved Table 6.2. Selected Standard Molar Enthalpies of
How do you calculate standard molar enthalpy of formation? Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. Definition and explanation of the terms standard state and standard enthalpy.
Using Standard Molar Enthalpies of Formation
Since we are adjusting the standard state thermochemical data from table 6.1 the lower. ∗the standard entropy of the h+(aq) ion is defined to be 0. Definition and explanation of the terms standard state and standard enthalpy of formation, with. How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy.
∗The Standard Entropy Of The H+(Aq) Ion Is Defined To Be 0.
How do you calculate standard molar enthalpy of formation? Definition and explanation of the terms standard state and standard enthalpy of formation, with. Since we are adjusting the standard state thermochemical data from table 6.1 the lower. Definition and explanation of the terms standard state and standard enthalpy of formation, with.