What Contaminates Annode At Discharge - In free flowing water or in very wet soil ground beds, there is very little restriction on current density. Even a fully charged cell deteriorates gradually. However, anodes buried in clay soils. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. But increased discharge increases the growth of precipitates.
However, anodes buried in clay soils. But increased discharge increases the growth of precipitates. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. In free flowing water or in very wet soil ground beds, there is very little restriction on current density. Even a fully charged cell deteriorates gradually. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig.
The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. However, anodes buried in clay soils. In free flowing water or in very wet soil ground beds, there is very little restriction on current density. But increased discharge increases the growth of precipitates. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. Even a fully charged cell deteriorates gradually.
(a) Picture of the pin liquid anode discharge taken using a camera and
The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. But increased discharge increases the growth of precipitates. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. Even a fully charged cell deteriorates gradually. However, anodes buried in.
Table 1 from Investigation of the Effect of Anode Fuel Contaminants on
But increased discharge increases the growth of precipitates. However, anodes buried in clay soils. Even a fully charged cell deteriorates gradually. In free flowing water or in very wet soil ground beds, there is very little restriction on current density. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble.
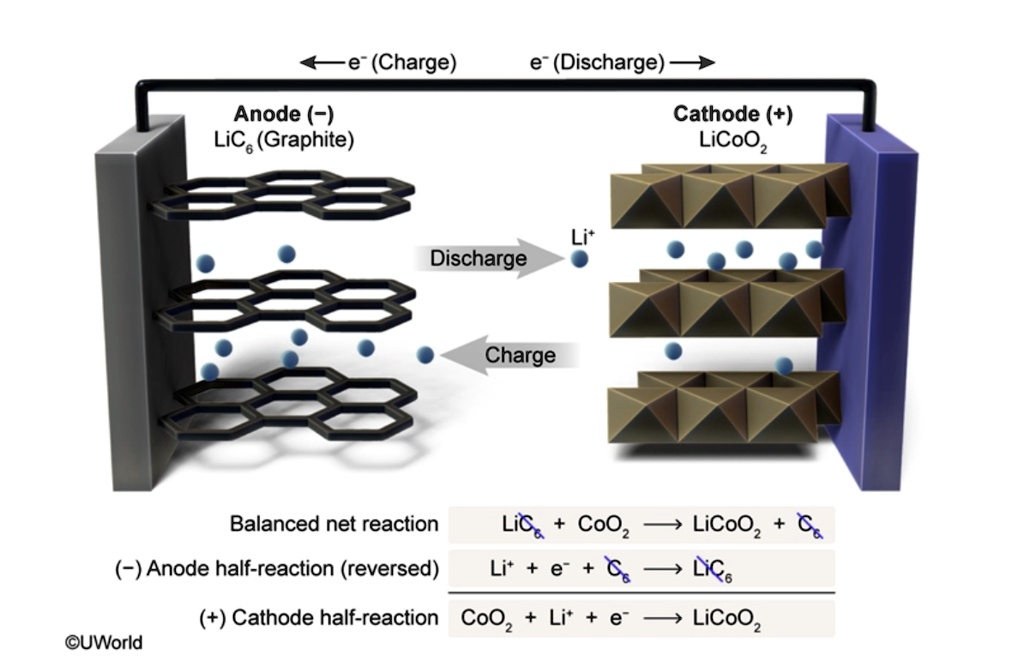
SOLVED (Charge) (Discharge) Anode ( ) Lics (Graphite) Cathode
However, anodes buried in clay soils. But increased discharge increases the growth of precipitates. Even a fully charged cell deteriorates gradually. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. In free flowing water or in very wet soil ground beds, there is very little restriction on current density.
Chargedischarge profiles for first cycle of the Si anode in 1 M LiPF 6
In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. Even a fully charged cell deteriorates gradually. In free flowing water or in very wet soil ground beds, there.
Principle setup of a battery cell with cathode, anode and separator
In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. Even a fully charged cell deteriorates gradually. However, anodes buried in clay soils. But increased discharge increases the growth of precipitates. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown.
Anode discharge VI characteristics for the UoSHHC and JPLHC 1
Even a fully charged cell deteriorates gradually. But increased discharge increases the growth of precipitates. However, anodes buried in clay soils. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. In free flowing water or in very wet soil ground beds, there is very little restriction on.
Discharge curves of the investigated anodes (a) Mg6Al, (b
But increased discharge increases the growth of precipitates. However, anodes buried in clay soils. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. In free flowing water or.
(a) Charge/discharge curve of an anode halfcell, with the anode behind
In free flowing water or in very wet soil ground beds, there is very little restriction on current density. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig..
Typical visible changes occurring in the cathode and anode cells. The
But increased discharge increases the growth of precipitates. However, anodes buried in clay soils. Even a fully charged cell deteriorates gradually. In free flowing water or in very wet soil ground beds, there is very little restriction on current density. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble.
Sources of water pollution as freshwater contamination causes
In free flowing water or in very wet soil ground beds, there is very little restriction on current density. However, anodes buried in clay soils. The anode, also known as the negatively charged electrode, discharges lithium ions into the electrolyte as shown in fig. In addition, anion contaminates, such as f − from hf and pf 5, readily react with.
The Anode, Also Known As The Negatively Charged Electrode, Discharges Lithium Ions Into The Electrolyte As Shown In Fig.
Even a fully charged cell deteriorates gradually. In addition, anion contaminates, such as f − from hf and pf 5, readily react with lithium to form insoluble reaction products. But increased discharge increases the growth of precipitates. In free flowing water or in very wet soil ground beds, there is very little restriction on current density.